 Regulating The Bacterial Photosystem In Response To Changes In Light Intensity Regulating The Bacterial Photosystem In Response To Changes In Light Intensity
The blue light regulated antirepressor, AppA
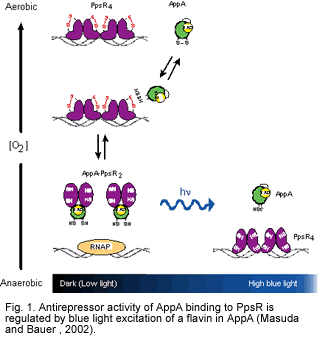
We intially described the existence of a blue light regulated transcription factor from Rhodobacter sphaeroides called AppA. AppA functions as an antirepressor of the redox controlled repressor called PpsR (PpsR is a homolog of CrtJ from R. capsulatus that is discussed in more detail the oxygen regulation section of our web site) (Masuda and Bauer 2002). Under dark or dim light conditions, AppA tightly binds to PpsR and inhibit the binding of PpsR to target DNA sequences. However, when illuminated with blue light, AppA is unable to bind to PpsR. The mechanism of light mediated regulation of AppA antirepressor activity is an active part of our research program and has been demonstrated by our group to involve light excitation of a flavin that is bound to AppA (Masuda and Bauer 2002)
Over the past number of years we have undertaken a combination of x-ray crystallographic, time resolved fast spectroscopic analysis using laser excitation and mutational analyses of AppA to delineate the mechanism of blue light regulation by AppA. These results have delineated conformational changes that occur in the flavin binding BLUF domain upon blue light excitation. Ultimately these conformational changes affect the ability of AppA to interact with PpsR.
Comparative Study With The Cyanobacterial Photoreceptor Slr1694
We have also undertaken a comparative approach toward understanding features of the AppA photocycle. For this analysis, we have obtained a crystal structure of PixD (Slr1694) from the cyanobacterium Synechocystis PCC6803. Like AppA, PixD is also member of the BLUF family of photoreceptors and as such has extensive homology to the BLUF domain of AppA. Mutations in PixD result in alteration of the blue light phototaxis (movement) response of Synechocystis indicating that it is part of the visual light perception machinery of cyanobacteria. Crystallographic analysis from our laboratory has demonstrated the PixD crystallizes as a stacked set of pentamer rings. We have also shown that a second protein called PixE is involved in forming these rings and that light excitation of PixD results in disassembly.
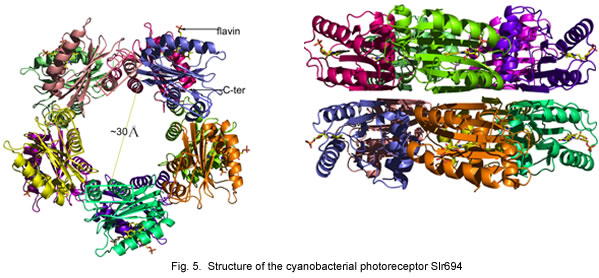
Individual subunits of Slr1694 show extensive similarity to the amino terminal domain of AppA.
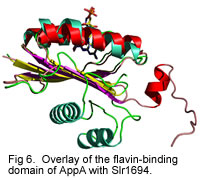
Currently we are undertaking crystallographic, mutational and spectroscopic analysis of AppA and Slr1694 to reveal features of the dark and light excited forms of these proteins. Our goal is to obtain an understudying of the molecular mechanism of the photocycle in this novel class of photoreceptors.
Light regulation using cobalamin (vitamin B12) as a photoreceptor
Recently we discovered a novel antirepressor of CrtJ called AerR that utilizes cobalamin (B12) as a cofactor (Cheng et al., 2014). Mutational studies with AerR shows that its ability to bind B12 is critical for the cells ability to control the synthesis of the photosystem in response to alterations in light intensity. This occurs because AerR only binds hydroxyl-cobalamin which is generated as a byproduct of light excitation of methyl-cobalamin, or adeno-cobalamin. Homologs of AerR are found in virtually all species of purple photosynthetic bacteria that also have homologs of CrtJ indicating that using B12 as a light sensor is a conserved mechanism. The significance of this study was highlighted by a commentary written buy G. Klug in the same issue of Mol. Micro.
References :
- Cheng, Z., K. Li, L. A. Hammond, J. A. Karty & C. E. Bauer. 2014. B12 controls photosystem gene expression by light regulated binding to the antirepressor AerR. Mol. Microbiol. In Press. NIHMSID 550433 (This work was highlighted in a companion commentary in the same issue by G. Klug.)
- Yin, L., V. Dragnea, G. Fieldman, L. A. Hammand, J. A. Karty, C. Dann & C. E. Bauer. 2013 Redox and light control of the heme sensing activity of AppA. mBio 4: e00563-13
- Yuan H, V Dragnea, Q Wu, KH Garner, & CE Bauer. 2011. Mutational and structural studies of the PixD BLUF output signal that affects light-regulated interactions with PixE. Biochemistry 50:6365-6375 PMID: 21688827
- Dragnea V, Arunkumar AI, Lee CW, Giedroc DP, & CE Bauer. 2010. A Q63E Rhodobacter sphaeroides AppA BLUF domain mutant is locked in a pseudo-light-excited signaling state. Biochemistry. 49:10682-90. PMID: 2108279
- Dragnea V, Arunkumar AI, Yuan H, Giedroc DP, & CE Bauer. 2009. Spectroscopic studies of the AppA BLUF domain from Rhodobacter sphaeroides: addressing movement of tryptophan 104 in the signaling state.Biochemistry. 48:9969-79. PMID: 19746968
- Yuan H. & CE Bauer. 2008. PixD promotes dark oligomerization of the BLUF photoreceptor PixD. Proc. Natl. Acad. Sci. USA 105, 11715-11719.
- Yuan H, S Masuda, V Dragnea, S Anderson, K Moffat and CE Bauer. 2006. Structure of the blue light photoreceptor, (Slr1694) from Synechocystis PCC6803 reveals photoinduced alterations of a hydrogen bond network to FAD. Biochemistry, 45: 12687-12694
- Dragnea V, M Waegelle, S Balascuta, CE Bauer and B Dragnea. 2005 Spectroscopic studies of the AppA blue-light receptor BLUF domain from Rb. sphaeroides in solution and in a crystal lattice with time-resolved spectroscopy. Biochemistry. 44:15978-15985.
- Anderson S, V Dragnea, S Masuda, J Ybe, K Moffat & CE Bauer. 2005. Structure of a novel photoreceptor: the BLUF domain of AppA from Rhodobacter sphaeroides . Biochemistry. 44, 7998-8005
- Kraft B, J Kikuchi, Masuda S, V Dragnia, G Tollin, JM Zaleski, & CE Bauer. 2003Spectroscopic and mutational analysis of the blue-light photoreceptor, AppA: A novel photocycle involving flavin stacking with aeromatic amino acids. Biochemistry , 42: 6726-6734
- Masuda S. & CE Bauer. 2005. The antirepressor AppA uses the novel flavin-binding BLUF domain as blue-light-absorbing photoreceptor to control photosystem synthesis. In Handbook of Photosensory Receptors. (W. Briggs and J. Spudich edt). Wiley-VCH publishing, Weinheim , Germany . pp. 433-446
- Masuda S & CE Bauer 2002 AppA is a blue-light photoreceptor that anti-represses photosynthesis gene expression in Rhodobacter sphaeroides Cell 110, 613-623. (Featured on cover)
|
